Supramolecular velcro unzipped by a voltage
Scientists in China have designed a velcro-like material held together by non-covalent interactions that can be unfastened by electrical means and refastened again under pressure.
The velcro is ‘stuck’ together by compressing a flexible, conductive poly(ionic liquid) membrane (PIL) functionalised with ferrocene (Fc) with a PIL functionalised with β-cyclodextrin (β-CD). The strong binding of the Fc groups within the β-CD cavities causes the layers to adhere together tightly. Oxidation of the Fc moieties to ferrocenium ions (Fc+) by chemical or electrochemical means causes the layers to come unstuck, as the charged Fc+ is not bound inside the hydrophobic β-CD cavity. A reducing potential and further pressing reassembles the material.
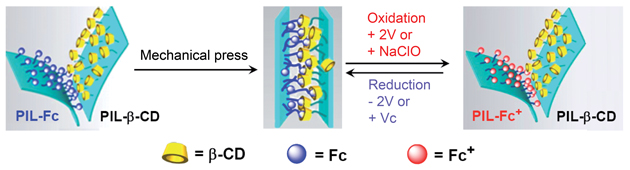
A hook-and-loop strategy fastens the layers together but these links can be unfastened by an electric current
The excellent properties of the velcro surprised even Feng Yan, whose team at Soochow University made the polymer, for two reasons: ‘Firstly, that a ~100mm thick velcro could withhold a 200g weight for more than 2 hours both in air and aqueous solutions, including acidic and basic water, and artificial seawater. And secondly, that such a polymer velcro could be cycled several times without obvious adhesion strength degradation.’ These properties could lead to potential applications that require controllable adhesion in aqueous environments, or in devices such as actuators in which a controlled response to an electrical current is key.
‘Although a similar supramolecular velcro based on cucurbituril and ferrocene has been reported recently, this new velcro has many additional interesting and significant features such as reversibility of the adhesion by applying a voltage, the use of a flexible conducting polymeric material and employing cyclodextrin as a macrocycle which is readily available and cheaper than the functionalised cucurbit[7]uril,’ comments Dönüş Toncel, who leads the polymer and supramolecular chemistry group at Bilkent University in Turkey.
Akira Harada, an expert in supramolecular chemistry and polymers from Osaka University in Japan thinks that the reported system will be interesting if it can be applied for medical treatment. Toncel also suggests that biomedical applications could facilitate the commercialisation of this new-generation velcro. ‘This is a very smart example demonstrating the realisation of supramolecular chemistry in a real-life application,’ she adds.
References
This paper is free to access until 19 June 2014. Download it here:
