Your Current Location is:Home > Research&Development
Progress in Research on Polarity of Ionic Liquids
New progress in the relationship between structure and polarity in ionic liquids (ILs) was achieved by the R&D Center for Green Chemistry and catalysis, Lanzhou Institute of Chemical Physics, CAS. The work was published in the recent issue of The Journal of Physical Chemistry B (J. Phys. Chem. B, 2010, 114 (11), pp 3912–3920. DOI: 10.1021/jp911430t).
Researchers revealed an interesting and unusual polarity behavior of hydroxyl ILs based on imidazolium moiety with various anions, wherein the hydroxyl groups exhibit a significant differentiating effect on their polarities. Polarity of nonhydroxyl ILs indicated by a series of solvatochromic dyes and fluorescence probe molecules exhibit anion-independent polarity, with similar ET(30) in the narrow range of 50.7–52.6 Kcal/mol, except [EMIm][AC] (49.7 Kcal/mol). However, the polarity of the hydroxyl ILs covers a rather wide range (ET(30) = 51.2–61.7 Kcal/mol) and is strongly anion-dependent. Kamlet-Taft parameters and density functional theory calculations indicated that the greatly expanded range of polarity of hydroxyl ILs is correlated to an intramolecular synergistic solvent effect of the ionic hydrogen-bonded HBD/HBA complexes generated by intrasolvent HBD/HBA association between the anions and the hydroxyl group on cations, wherein hydroxyl group exhibits a significant differentiating effect on the strength of H-bonding and thus the polarity. Spiropyran-merocyanine equilibrium acted as a model polarity-sensitive reaction indeed shows obviously polarity-dependent solvatochromism, photochromism, and thermal reversion in hydroxyl ILs.
Noteworthy is that the hydroxyl ILs of “hyperpolarity” in current research, which behaved as single-component substance and not as a mixture, will certainly fill the polarity gap between water and general organic solvents and could be employed as high polar nonacidic and nonaqueous ionic media for synthesis and catalysis, especially for the polarity-promoted applications. The work also confirmed the increased cation-anion interactions through introduction of a more active group and demonstrated the possibility of rational design and synthesis of polarity-specific ILs.
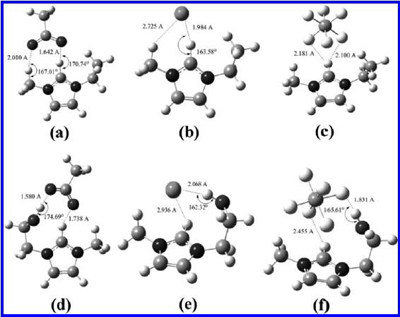
The optimized geometries of six ILs from B3LYP/6-31+g (d,p). (a) [EMIm][AC], (b) [EMIm][Cl], (c) [EMIm][PF6], (d) [HOEMIm][AC], (e) [HOEMIm][Cl], and (f) [HOEMIm][PF6] (H-bond is indicated as dashed line).