Your Current Location is:Home > Research&Development
Polymeric Ionic Liquid as a Dynamic Coating Additive for Separation of Basic Proteins by Capillary
Capillary electrophoresis (CE) has become an effective tool in the separation of charged and neutral compounds due to the various advantages it possesses. However, this technique for proteins analysis is subject to some critical limitations owing to the tendency of protein to be adsorbed onto the negatively charged surface of fused-silica capillaries. Recently, much attention has been paid to polymeric ionic liquid (PIL) that are described as a novel class of materials combining the properties of ionic liquids (ILs) and the specificities of polymers. However, the application of PIL in CE separation of proteins has not been reported.
Researchers of the Key Laboratory of Chemistry of Northwestern Plant Resources of the CAS have developed a simple and economical electrophoresis method for the analysis of basic proteins using the fused-silica capillary. A PIL, poly(1-vinyl-3-butylimidazolium) bromide, was used as the BGE additive in CE separation of basic proteins for the first time. This PIL had been proved to generate a strong reversed and stable EOF because of the capillary wall modification through electrostatic interaction between the cationic PIL and the negatively charged capillary inner surface.
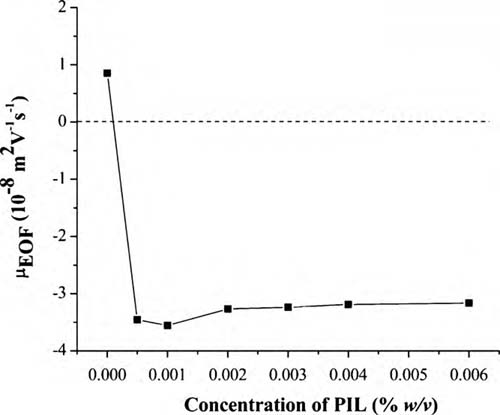
Effect of PIL concentration on EOF. Electrophoresis conditions: capillary, 48.5cm (40cm to detector) ×50 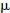 m i.d.; BGE, 60mM NaH2PO4 (pH 4.0) containing different concentration of PVBIm+ Br−; injection, 50 mbar 3 s; applied voltage,±20 kV; temperature, 25 ℃; detection, 200 nm.
m i.d.; BGE, 60mM NaH2PO4 (pH 4.0) containing different concentration of PVBIm+ Br−; injection, 50 mbar 3 s; applied voltage,±20 kV; temperature, 25 ℃; detection, 200 nm.
The results demonstrated that the repulsive interactions between the positively charged PIL and the basic proteins effectively eliminate the adsorption of the basic proteins onto the capillary wall. Under the optimum conditions, the capillary dynamically modified with PIL exhibited baseline separation, good repeatability and high efficiencies for four basic proteins.
This method is economical, simple, and requires no complex chemistry. It also broadens the application of the IL in CE separation.
The work was supported by the National Natural Science Foundation of China and was published in Analytica Chimica Acta (Analytica Chimica Acta 674 (2010) 243–248).