LANL takes on deadly bugs

Team members who discovered a treatment for resistant infections are, from left, Aimee Newsham, Dixie State University student; Rico Del Sesto, Dixie State University professor; David Fox, Los Alamos National Laboratory; Andrew Koppisch, Northern Arizona University professor; and Mattie Jones, Dixie State University student. (Courtesy of David Fox)
This column is for everyone who has ever had to deal with a horrible infection – from the Streptococcus sp. that rots teeth, to the Pseudomonas aeruginosa that attacks diabetic patients’ feet, to the Methicillin-resistant Staphylococcus aureus (MRSA, i.e. flesh-eating bacteria) that makes any original medical problem much worse. And it’s for everyone who ever will.
Considering a surgeon recently told me MRSA is our generation’s staph, in that it’s everywhere and on everything, that could be everybody.
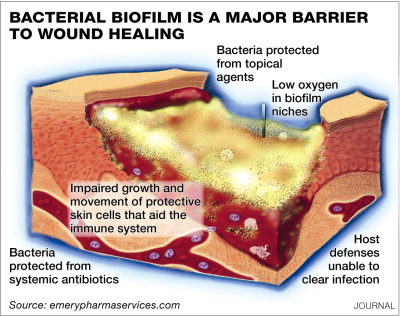
Treating these infections, known in the scientific world as biofilms, is expensive, time consuming, sickening and often unsuccessful when it comes to killing them before they kill their host. That’s because while they are responsible for up to 80 percent of all bacterial infections, they have their own protection that makes them 50 to 1,000 times more resistant to antibiotics.
And that’s why a discovery at Los Alamos National Laboratory – a treatment with what amounts to fancy water – is beyond exciting. It could be life-changing and life-saving.
Full disclosure: My mother contracted a biofilm infection after having a back surgery and spent years unsuccessfully fighting it with stronger and stronger oral and intravenous antibiotics that ultimately caused a serious reaction of their own. The drugs simply could not penetrate the infection to kill it. Doctors finally decided to remove the hardware (and its virulent bacteria) rather than continue a fruitless and damaging battle.
In David Fox’s world, my mother would have had an antibiotic delivered via ionized liquid that could penetrate her skin, the biofilm, and kill the bug.
Fox is a staff scientist in LANL’s Bioscience Division. For several years he and a team of fellow chemists and microbiologists have been working with ionic liquids – known as molten salts. Originally their work was for forensic applications, like how to pull certain molecules out of fabrics. The team then figured out they could also use the ionic liquids to deliver molecules: like antibiotics to an until-then impenetrable bacteria.
So these scientists – Fox, Tari Kern, Katherine Lovejoy, Rico Del Sesto (now at Dixie State University), Rashi Iyer, Amber Nagy, Andrew Goumas, Tarryn Miller and Andrew Koppisch (now at Northern Arizona University) – started working with the University of California-Santa Barbara on using their ionized water for transdermal drug delivery.
Instead of infection treatments that range from irritating to painful – organic solvents, injections and debridement – the team focused on using 12 ionic liquids “generally recognized as safe” (GRAS in science-speak). They grew opportunistic gram negative bacteria, then added individual ionic liquids and incubated, then rinsed.
And what they found was a greater than 99.9999 percent bacteria cell death, with some of the ionic liquids “more effective than a 10 percent bleach solution.”
And that was before adding antibiotics.
The team then moved on to ensuring the liquids with dissolved antibiotics could penetrate pig skin and the bacteria’s protective layer – and got equally stunning results. “Ninety-five and 98 percent reduction in cell viability” with one of the ionic liquids and that liquid plus an antibiotic.
By comparison, antibiotics alone had a 20 percent kill rate.
So why should someone who’s never had a cavity or a diabetic ulcer or a MRSA infection care? Fox points out the “economic burden of skin disease is over $100 billion.” That MRSA-type infections acquired in hospitals account for an estimated “$10 billion in extra patient costs and over 10,000 deaths per year.” That “wounds from infected surgical incisions account for over 1 million additional hospital days.” And that 10 to 20 percent of diabetic ulcers – a function of the Pseudomonas aeruginosa infection – require amputation.
In other words, we are all paying for it, in terms of money, health and life.
The discovery is now moving into clinical studies with live subjects – mice – Fox says, and if those go as well, on to human clinical trials. Funding for the years of required additional research could come from energy companies that want to extract high-energy density molecules like biofuels from an organism (the research’s first application), from corporations that could use it to more efficiently deliver their drugs to patients, and/or from the military that wants to protect/treat its soldiers.
“Thousands of people die from, and billions is spent on treating, these secondary infections,” Fox says. The LANL team could be “providing a new weapon to combat flesh-eating bacteria and other microbes. We hope we have found a new silver bullet to treat these infections. We hope that’s where we’re at.”
And so does everyone who has had, or will get, one of these very nasty infections.
UpFront is a daily front-page news and opinion column. Comment directly to assistant editorial page editor D’Val Westphal at 823-3858 or road@abqjournal.com. Go to ABQjournal.com/letters/new to submit a letter to the editor.