Driving towards success with biomass-derived petrol
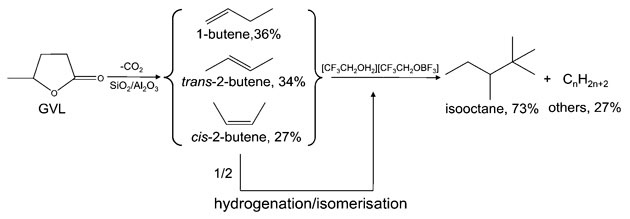
Process for converting GVL into high octane number petrol
Chinese scientists have overcome previous limitations to generate high octane number petrol from biomass-derived γ-valerolactone (GVL), an organic compound that is already often blended in small amounts with petrol or diesel. Using an ionic liquid catalyst, the conversion churned out a 2,2,4-trimethylpentane-rich substance with an octane number of 95.4, the highest reported for biomass derived fuel.
Petrol, the liquid many of us use to run our cars, is typically obtained from fossil fuels. But, with energy demands rocketing, producing a renewable and sustainable alternative has become a challenge for many researchers.
Although the concept is not new, previous biomass-derived fuels have only seen success as substitutes for jet fuel additives or diesel. This is because the resultant alkanes are linear or have a single branch, ‘making them incompatible with those used in the petroleum industry,’ explains Suojiang Zhang from the Institute of Process Engineering at the Chinese Academy of Sciences, Beijing, who led the work. ‘Reports on high octane number gasoline from biomass are very rare,’ he adds.
This is linked to a fundamental property of petrol, the anti-knock index, a measure of resistance to ignition best indicated by the research octane number (RON) and achieved through the presence of highly branched C8 alkanes.
And Zhang and his group have made just this, producing a fuel which meets European requirements for RON and surpasses those for lead, sulfur, olefin, aromatic and benzene levels, without the potentially toxic additives usually needed.
After generating GVL from commonly available lignocelluloisic biomass it undergoes decarboxylation to produce butenes, some of which are converted to isobutane. This is followed by alkylation using an ionic liquid catalyst, [CF3CH2OH2][CF3CH2OBF3], to give the isooctane product.
The key to the method’s success and arguably the biggest challenge is in the design of an ionic liquid catalyst with suitable acidity. Cracked products result if the acid strength is too high whereas oligomerisation to higher carbon fractions occurs when it is too low. The process is thus catalysed by protons in a chain-like manner. As a liquid catalyst, [CF3CH2OH2][CF3CH2OBF3] has sufficient active sites to ensure good activity and, due to its ionic nature, can be easily removed by phase separation. In short, a huge improvement without the need for hydrodeoxygenation and additives.
Green chemistry expert Liang-Nian He, from Nankai University in China, describes the work as a significant breakthrough that will ‘stimulate further interest in more cost-efficient processes to produce biomass-based gasoline on a larger scale’.
Indeed, this is what the group aim to do: whilst rolling out plans to scale up this valuable method they are continuing to develop more efficient and feasible routes to produce high quality fuels.
References
This article is free to access until 22 December 2014. Download it here:
