Developments in Gas Chromatography Using Ionic Liquid Stationary Phases
ionic liquids (ILs) have become recognized in gas chromatography (GC) as stable and highly polar stationary phases with a wide application range. Having customizable molecular structures, ILs also offer a particular tunability that provides additional selectivity, and therefore may improve separation for neighbouring analytes. This article presents specific properties of IL phase capillary GC columns, including polarity scale and inner surface morphologies of IL columns. Application of IL phases in achiral and chiral GC, and multidimensional GC, are highlighted.
The column plays a pivotal role in the quality of the results obtained in gas chromatography (GC). The physical dimensions of the column, the type of coated stationary phase used, the operating temperature range of the stationary phase, the retention adjustment of relative peak positions based on “phase polarity”, and the longevity of the column to ensure consistent results are all important considerations to achieve satisfactory separation efficiency and performance in GC.
Most recent developments in columns for GC have focused on improved selectivity and improved inertness of GC capillary stationary phases. Thus, any developments related to the introduction of a new type of stationary phase in the form of ionic liquid (IL) capillary GC phases opens up new possibilities for GC separations.
Ionic Liquid Phases in GC
Separation in GC with conventional stationary phases, such as poly(siloxane) and poly(ethylene glycol), is mainly achieved as a result of the vapour pressure and polarity difference between the separated compounds and phases. This often limits the separation power for target regions, and the chemical interactions that can be exploited in one‑dimensional GC. Specialty phases such as those based on liquid crystals (with structural selectivity) and chiral phases (to resolve optical isomers) are examples that offer separations not possible on other phases. The number of stationary phases that can be effectively applied in multidimensional GC (MDGC), where at least two columns are used to increase the number of compounds that can be separated (and ideally identified) in an analysis, is also limited because of a lack of selectivity variation amongst available columns, and some compounds might not be adequately separated.
To overcome these limitations, additional types of interactions between the analytes and stationary phases are required. If a phase can allow new types of interactions between solutes and the phase through the chemical construction of the phase then a new mechanism of retention will emerge; ionic liquid (IL) phases aim to provide this opportunity for new separation mechanisms in GC.
ILs — salts in a liquid state — have found a wide range of applications in chemistry including analytical chemistry, mainly because of their characteristic adaptable properties (1). The potential “green” application of ILs as solvents to dissolve almost any chemical was identified as the innovation “most likely to shape the 21st century” (2). The combination of hundreds of cationic and anionic species enables the tuneability of nearly every important property of the IL material, from thermal stability, variable dielectric constant of the medium, to the aggregation state. In addition, molecular and physical features can be designed and modulated to incorporate various degrees of biodegradability, biocompatibility, and reusability, to extend their application from chemical, biological, environmental, and medicinal applications. Some of the most common applications of ILs are summarized in Figure 1.
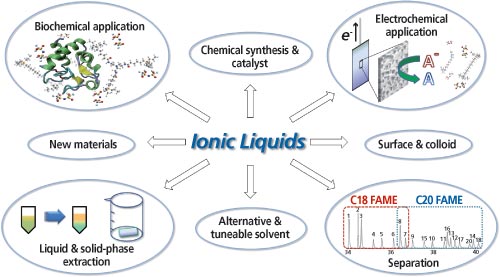
Figure 1: Scope of applications related to ionic liquids.
ILs are attractive stationary phase materials for GC, offering several benefits, including high polarity, high viscosity, and low vapour pressure as a result of their ionic nature, which helps to improve GC column coating. The chemical flexibility of their organic part, and thermal stability of their inorganic part (3) all offer great potential for R&D researchers to develop new stationary phases based on ILs.
Ionic Liquid Phase Capabilities
Reliable comparison of many conventional and IL stationary phases has been documented (3). A lack of hydrogen-bond acidity (affinity towards basic analytes) with conventional GC stationary phases has been noted, and the observed hydrogen-bond acidity of several ILs indicates that additional selectivity may arise from use of an IL-based material, which in turn offers a degree of orthogonality (a parameter representing distribution of analyte peaks in MDGC) not accessible with other phase coatings. This will be discussed in more detail later in this article.
The benefits of IL column applications are expected in a variety of separations, especially when higher polarity and higher temperature separation is required. Polarity of phases can be scaled by the well-known McReynolds constant values (higher values indicating higher polarity of the phase). IL phases (Supelco) with their respective McReynolds polarity (P) are reported in Table 1 (4,5).

IL phases will increasingly retain more polar solutes compared with less polar solutes, or compounds with functional groups that provide specific enhanced interactions with the phase such as unsaturated carbon–carbon bonds. IL phases also provide improved tuning power in separation and resolution of some isomers that may be difficult to obtain by using conventional phases.
For the remainder of this article, the IL phases will be referred to on the basis of their polarity, for example, IL2622, IL4437, or as appropriate, to provide a generic basis to the role of IL phase polarity.
Ionic Liquid Phase Applications
Some commercialized phosphonium and imidazolium based IL columns (6) have been applied for the separation of many samples such as fatty acid methyl esters (FAMEs), chlorinated hydrocarbons, essential oils, pesticides, and related compounds in different sample matrices. They have been most commonly used as one‑dimensional (1D) GC columns, but IL phases may also be incorporated into multidimensional (MD)GC either as the first (1D) or second (2D) dimension column; they also offer increased selectivity in 3D separation (3). Interestingly, different IL columns reportedly have specific selectivity or application towards different analytes (3). A snapshot of the application areas where IL stationary phases could provide additional selectivity, as well as improved separation outcomes, compared with classical wall-coated open-tubular column stationary phases, is summarized in Figure 2 according to the literature (3). Based on the P scale, IL2622 is suitable for the increased retention and improved separation of several compounds in essential oils such as alcohols and aromatic esters; IL2624 is effectively applied for the analysis of benzothiazoles, benzotriazoles, and nitrosamines in wastewater samples. One-dimensional separation of 106 tetra- to octa-chlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) congeners was obtained by using IL2710; whilst improved separation of PCDD/Fs congeners is expected with the combination of IL3379 and a smectic biphenyl carboxylate ester liquid crystalline polysiloxane column. IL3638 was able to resolve galaxolide isomers. IL4437 is preferred for the separation of alkyl phosphates in petroleum samples. IL4437 and IL4938 are now well known for FAME separation. In all these cases, these applications will not necessarily be specific to (that is, only possible on) the indicated column, but rather have been usefully employed for the specific target application. In addition, thermal stability should also be noted for the selection of IL columns. The temperature limit values for some IL columns are provided in the caption to Figure 2.
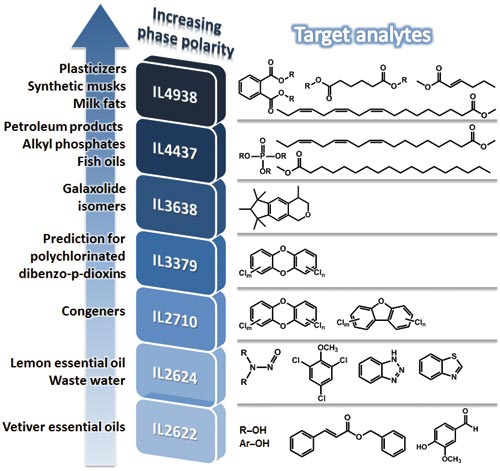
Figure 2: Selected applications of IL columns with different McReynolds polarity (P) in GC for separation of different specific target compounds. IL2622 and IL2624 = 1,12-di (tripropylphosphonium)dodecane [Tf2N], IL2710 = 1,12-di (tripropylphosphonium) dodecane [TfO], IL3379 = tri (tripropylphosphoniumhexanamido)triethylamine [Tf2N], IL3638 = 1,12-di(2,3-dimethylimidazolium)dodecane [Tf2N], IL4437 = 1,9-di(3-vinylimidazolium)nonane [Tf2N], IL4938 = 1,5-di(2,3-dimethylimidazolium)pentane [Tf2N], where the anions [Tf2N] = bis(trifluoromethylsulphonyl)imide and [TfO] = trifluoro methyl-sulphonate. The corresponding temperature limits for columns prepared using these phases are reported to be 300, 300, 290, 270, 270, 230, and 270 °C, respectively.
Tuneable separation results have also been noted. For example, a set of IL columns with increasing polarity provides a tuneable capability to separate the group of C18 FAMEs, or the C20 FAME group (4). Therefore IL2622, IL3638, and IL4938 result in a progressively decreased extent of separation (and increased overlap) of these FAME groups (Figure 3). A higher polarity IL column correlates to better separation within each FAME group (that is, more separation between unsaturated and saturated FAMEs in that group), but, consequently, there is a greater intersection or overlap between the C18 and C20 FAME groups, which is maximized for IL4938.
The Surface of the Ionic Liquid-Coated Capillary
Scanning electron microscopy (SEM) allows investigation of inner wall surface morphologies of IL columns. Unlike an expected surface morphology of the IL capillary inner wall providing a smooth and homogeneous IL film, in a SEM study of the inner wall we observed that the surface adopts a somewhat microscopic surface particulate topology (Figure 3). This study did not use a metal coating deposited on the wall surface as normally employed prior to analysis by SEM. However, it should be noted that a uniform distribution of the IL material over the capillary inner walls was still clearly seen. Golay suggested that, contrary to a column phase presenting a smooth surface, should a uniform (but rough) inner wall surface of the open tubular column be formed, it is still possible to result in high performance separations (7). This might be similar to that produced by a thin layer of graphitized carbon black as the stationary phase support for capillary columns in GC. This is consistent with the excellent separation obtained according to the stable and uniform inner wall surfaces of the columns.
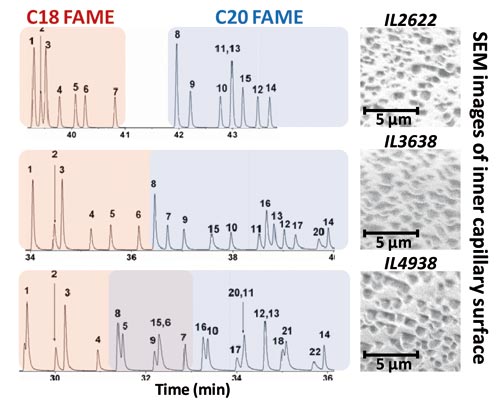
Figure 3: 1D-GC chromatograms of C18 and C20 FAME (red and blue regions, respectively) and SEM images of the inner capillary surfaces for IL2622, IL3638, and IL4938 (without a deposited metal coating) obtained at the Monash Centre for Electron Microscopy.
Application of Ionic Liquid Phases in Chiral GC
There are two possibilities for the application of ILs in chiral GC. A chiral selector can be dissolved in an achiral IL to allow chiral recognition between the selector and the target analytes. Alternatively, the IL itself can be synthesized to incorporate chirality by addition of chiral cation motifs into IL molecules (3). Incorporation of cyclodextrin functionality into the cation component of IL material is reported and is highlighted here as the intersection between IL with conventional cyclodextrin. A good example is the permethyl-6-(butylimidazolium)-β-cyclodextrin iodide IL stationary phase, which has been reported to offer better chiral separation performance than comparative conventional phases, particularly for the separation of highly polar racemates (8). Another example is the chiral N,N-dimethylephedrinium IL stationary phase that indicated improved separation of polar enantiomers such as acetylated amines, alcohols, epoxides, and sulphoxides (9). The scope for innovation in chiral analysis based on IL is clearly substantial, and one can anticipate continuing activity here.
Ionic Liquid Phases and Advanced Methods in GC (MDGC and GC×GC)
While IL phases have useful selectivity, this does not increase the peak capacity per se for analysis of a given sample. This is akin to “rearranging the deck chairs on the Titanic” — it merely shuffles the positions of peaks, but complex samples may still consist of largely unresolved regions of peaks. If the column provides much narrower peaks and/or extends the thermal range of the application, this may lead to a larger number of resolvable peaks. But there is no reason to think that an IL phase can be made “better” in terms of efficiency (plates per m) of the separation process. So, for complex samples (that is, those with a large number of components) it is still important to consider the use of hyphenated arrangements for columns in the separation experiment, such as two-dimensional heartcutting GC (GC–GC), or comprehensive 2D GC (GC×GC) (10,11).
The use of a highly selective IL column (phase) in GC–GC to transfer the target zone, where either the 1D or 2D column comprise an IL, or where one or possibly both dimensions of a GC×GC method incorporates an IL column, has been increasingly reported for a range of applications in the literature as illustrated for separation of FAME, allergens, aromatic hydrocarbon, polychlorinated biphenyl, pesticides, N-and S-containing compounds in different sample matrices (3,12,13).
ILs have become increasingly attractive for applications in multidimensional GC because of their ability to provide additional separation mechanism(s) and selectivity in separations (3,12,13). IL columns also offer the capability to retain non-polar analytes (even better than some conventional polar phases) as demonstrated for kerosene separation; for example, when using trihexyl(tetradecyl)phosphonium tetrachloroferrate with the impressive maximum allowable operating T at 320 °C — higher than all the commercial IL columns and the conventional PEG phase (12). This surprising observation counteracts the claim that the high polarity of IL phases could lead to weak (poor) retention of non-polar analytes in 2D separation, implying that the distribution constant favours the stationary phase for the non-polar analytes, although not as much as polar analytes.
Furthermore, regarding the benefit of customizable molecular properties, obtaining tuneable separation results is relatively straightforward; simply by choosing a column with a larger difference in polarity (ΔP) a better peak distribution of FAME in GC×GC can be obtained (Figure 4). It is also possible to predict 2D separation results by variations of IL molecular structures; this could ultimately guide synthesis of novel IL phases for separation of specific target analytes in the future (14). This is a powerful and unique property of IL phases; the potential combination of specific cations and anions to provide predictable and unique separation performance, ideally modelled by suitable computer optimizations, will permit a hitherto unexplored opportunity for advanced separations research.
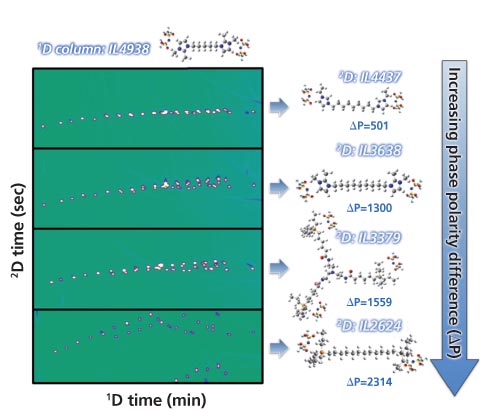
Figure 4: Molecular structures of different IL phases and their GC×GC chromatograms of 37 FAME mixture for 2D column being IL2624, IL3379, IL3638 and IL4437 with IL4938 fixed as the 1D column. Adapted and reproduced with permission from Journal of Chromatography A 1312, Asia Nosheen, Blagoj Mitrevski, Asghari Bano, and Philip J. Marriott, Fast comprehensive two-dimensional gas chromatography method for fatty acid methyl ester separation and quantification using dual ionic liquid columns, 118–123 (2013) © Elsevier.
This indicates that IL phases have a potential to move target analytes within the chromatographic space, and if this can be based on an informed prediction model, a powerful tool to optimize separation results will be in the chromatographers’ hands. More interestingly, 1,5-di(2,3-dimethylimidazolium)pentane bis(trifluoromethylsulphonyl)imide also possesses high thermal and surface sensitivity, which apparently leads to variable selectivity; this has the novel and surprising outcome of providing good 2D separation of FAMEs by using a sole single IL stationary phase (that is, same phase in both dimensions), which we have termed “1-phase-GC×GC” (15).
results by variations of IL molecular structures; this could ultimately guide synthesis of novel IL phases for separation of specific target analytes in the future (14). This is a powerful and unique property of IL phases; the potential combination of specific cations and anions to provide predictable and unique separation performance, ideally modelled by suitable computer optimizations, will permit a hitherto unexplored opportunity for advanced separations research.
This indicates that IL phases have a potential to move target analytes within the chromatographic space, and if this can be based on an informed prediction model, a powerful tool to optimize separation results will be in the chromatographers’ hands. More interestingly, 1,5-di(2,3-dimethylimidazolium)pentane bis(trifluoromethylsulphonyl)imide also possesses high thermal and surface sensitivity, which apparently leads to variable selectivity; this has the novel and surprising outcome of providing good 2D separation of FAMEs by using a sole single IL stationary phase (that is, same phase in both dimensions), which we have termed “1-phase-GC×GC” (15).
Conclusions
IL phases represent an interesting, high-polarity, thermally-stable, tuneable, and novel class of GC phase. They have been demonstrated to provide useful selectivity for separation of a growing portfolio of samples; the opportunity to expand this to further fundamental research, to better characterize the range of molecular interactions that can be accessed through novel IL chemical designs, and then to apply IL phases to a much wider range of applications, is anticipated in the near future.
References
- 1. J.P. Hallett and T. Welton, Chem. Rev. 111, 3508–3576 (2011).
- 2. E. Stoye, Ionic liquids win Great British Innovation Vote. http://www.rsc.org/chemistryworld/2013/03/great-british-innovation-vote-... (accessed 14/11/2014).
- 3. C.F. Poole and N. Lenca, J. Chromatogr. A 1357, 87–109 (2014).
- 4. A.X. Zeng, S.-T. Chin, Y. Nolvachai, C. Kulsing, L.M. Sidisky, and P.J. Marriott, Anal. Chim. Acta 803, 166–173 (2013).
- 5. Supelco Supelco Ionic Liquid GC Columns - Introduction to the Technology. https://www.sigmaaldrich.com/content/dam/sigma-aldrich/countries/czech/g... (accessed 14/11/2014).
- 6. D.W. Armstrong and Z.S. Breitbach, Anal. Bioanal. Chem. 390, 1605–1617 (2008).
- 7. T. Halasz and C. Horvath, Nature 197, 71–72 (1963).
- 8. K. Huang, X. Zhang, and D.W. Armstrong, J. Chromatogr. A 1217, 5261–5273 (2010).
- 9. J. Ding, T. Welton, and D.W. Armstrong, Anal. Chem. 76, 6819–6822 (2004).
- 10. S.T. Chin and P.J. Marriott, Chem. Commun. 50, 8819–8833 (2014).
- 11. P.J. Marriott, S.T. Chin, B. Maikhunthod, H.G. Schmarr, and S. Bieri, TrAC Trends Anal. Chem. 34, 1–20 (2012).
- 12. L.W. Hantao, A. Najafi, C. Zhang, F. Augusto, and J.L. Anderson, Anal. Chem. 86, 3717–3721 (2014).
- 13. A. Nosheen, B. Mitrevski, A. Bano, and P.J. Marriott, J. Chromatogr. A 1312, 118–123 (2013).
- 14. C. Kulsing, Y. Nolvachai, A.X. Zeng, S.-T. Chin, B. Mitrevski, and P.J. Marriott, ChemPlusChem 79, 790–797 (2014).
- 15. Y. Nolvachai, C. Kulsin, and P.J. Marriott, Anal. Chem. 87, 538–544 (2015).
Philip Marriott is a Professor in the School of Chemistry, Monash University, Australia, and Deputy Director of the Australian Centre for Research on Separation Science (ACROSS). Previous appointments were at University of Bristol, National University of Singapore, and RMIT University. He is active in high resolution separations and leads research programmes in GC×GC and MDGC. He has published about 330 research papers and book chapters.
Chadin Kulsing is a postdoctoral researcher in the group of Prof. Marriott. His research area covers thermodynamics, kinetics, surface, and solution chemistry in chromatography, and he is currently focusing on a computational approach for understanding separation mechanisms in MDGC and ion fragmentation in MS. He has published 19 research papers and book chapters.
Yada Nolvachai commenced her PhD candidature in September 2012, in the School of Chemistry, Monash University, with Prof. Marriott. Her current PhD research focuses on software development based on thermodynamics and quantum mechanics for novel material selection in multidimensional gas chromatography. She has published 13 research papers and book chapters.
Helmut Hügel is Assoc. Professor in the School of Applied Sciences at RMIT University. He is an organic chemist by training, and has collaborated with Prof. Marriott on a variety of separation science and synthetic projects, including SPME, development of ionic liquid phases, interconversion processes in GC, and marine fragrance synthesis. He has published about 110 research papers and book chapters.
